-
PRODUCTS
- Anaesthesia & Respiratory
- Baby
- Casting
- Continence Care
-
Diagnostic
- See all Diagnostic
- Accessories
- Bladder Scanner
- Blood Testing
- Breathalyzer
- Cholesterol Testing
- Covid Testing
- Dermatoscope
- Diabetes Monitoring
- Drug Testing
- Endoscopy
- Eye Ear Nose & Throat
- Height Measures and Charts
- INR
- Jars and Containers
- Medical and Surgical Instruments
- Parts and Accessories
- Scales
- Testing
- Urinalysis
- Womens Health
- Ear Irrigation
-
Equipment
- See all Equipment
- "Miscelllaneous, Parts and Accessories"
- Beds
- Blood Collection Chair
- Bracket & Dispensers
- Carts & Trolleys
- Cryosurgery
- Electrosurgery
- Examination Couches & Tables
- Fridge & Freezers
- IV Stands
- Laundry & Cleaning Trolleys
- Miscellaneous
- Play Panels
- Privacy Screens and Curtains
- Stools and Step Ups
- Tens
- Transferring & Patient Handling
- Waste Disposal
- X-Ray Viewers
-
General Consumables
- See all General Consumables
- "Couch Rolls, Protectors & Underpads"
- "Registers, Records and Certificates"
- Bags Assorted
- Batteries
- Blankets
- Blankets and Warmers
- Bracket
- Child Rewards
- Containers
- Cups
- Dr Bags
- Ear Piercing
- Episcope
- Feminine Hygiene
- First Aid and Trauma
- Gels and Lubricants
- Identification
- Linen
- Marker
- Miscellaneous
- Ostomy
- Paper and Printing Consumables
- Paper Products
- Parts and Accessories
- Personal Care
- Pill Cutters and Crushers
- Ultrasound Gel
- Wooden Applicators
- Gloves
- Hand & Body Hygiene
-
Infection Prevention & Control
- See all Infection Prevention & Control
- Absorbent Powder
- Bedpans & Urinals
- Caps
- Containers
- Dispensers
- Eye Protection
- Face Masks
- Hand Hygiene
- Miscellaneous
- Parts & Accessories
- Protective Apparel
- Scrubs
- Spill Kit
- Surface Cleansers & Wipes
- Surgical Packs & Drapes
- Toileting & Waste Disposal
- Tray Liners
- Wipes and Skin Protection
- Intravenous Infusion & Administration
-
Medical & Surgical Instruments
- See all Medical & Surgical Instruments
- Biopsy Punch
- Chiropody Pliers & Podiatry
- Cleaning and Protection
- Curettes
- Dental Syringe
- Dilator
- Ear Irrigation
- Forceps
- Hammer
- Male Health
- Marker
- Miscellaneous
- Nasal Speculum
- Needle Holder
- Pack
- Probe
- Retractor
- Ring Cutter
- Scalpel Handles & Blades
- Scissors
- Skin Hook
- Sucker
- Tuning Forks
- Uterine Curettes & Sounds
- Medical Lighting
- Medical Lighting
- Needles & Syringes
- Nutritional Support
- Oral Care
- Patient Monitoring
-
Pharmaceuticals
- See all Pharmaceuticals
- Alimentary
- Anaesthetic
- Analgesia
- Antihistamines
- Cardiovascular
- Central Nervous System
- Creams and Ointments
- Endocrine & Metabolic
- Eye Ear Nose & Throat
- Infections & Infestations
- Miscellaneous
- Musculoskeletal
- Nutrition
- Ointment Products
- Other
- Register
- Respiratory
- Skin
- Solutions
- Rehabilitation & Mobility
- Skin Care
- Sports & Recovery
-
Sterilisation
- See all Sterilisation
- Autoclaves
- Biological Indicators and incubators
- Chemical Indicator Tapes
- Chemical Indicators and Integrators
- Cleansing Solutions & Detergents
- Instrument Protector
- Labels
- Marker
- Paper and Printing Consumables
- Parts & Accessories
- Record Keeping Supplies
- Steam Indicator Sheets and Tests
- Sterilisation Pouches & Rolls
- Towels and Cloths
- Trays and Bowls
- Ultrasonic Cleaners
- Water
- Wraps
- Sutures & Skin Closures
- Urology
- Vaccines
- Wound Care
- Wound Management
- News & Updates

Sodium Chloride 0.9% Dual PVC 1000ml - Box/10
P.O.A.
Code: 10022743
Supplier Part: AB-PAR-S19-04
Unit: BOX 10
The above Australian registered medicine is in shortage due to an unexpected increase in consumer demand. Aborns Pharmaceuticals Pty Ltd has been able to arrange for supply of the following alternative product, registered and marketed in France, on a temporary basis:
Sodium Chloride 0.9% Bioluz, Solution for Infusion in Dual Access PVC Bag 1000ml (France). This product is NOT registered in Australia and supply is authorised under an approval granted by the Therapeutic Goods Administration (TGA) under section 19A of the Therapeutic Goods Act 1989 until 30 April 2025 for the following indications:
- for extracellular fluid replacement and in the management of metabolic alkalosis in the presence of fluid loss, and for restoring or maintaining the concentration of sodium and chloride ions.
The s19A approved product is identical in active ingredient and strength to the Australian registered product. It is registered and marketed in France and therefore, all the labelling is in the French language. The name of the medicine and strength is identifiable in the English language to Australian Healthcare Professionals. The package leaflet is in the French language. The s19A medicine is in the form of solution for infusion bag with dual access, equipped with a collection site and an infusion site.
The key differences between the Australian registered products and the France registered products are outlined in the table below:
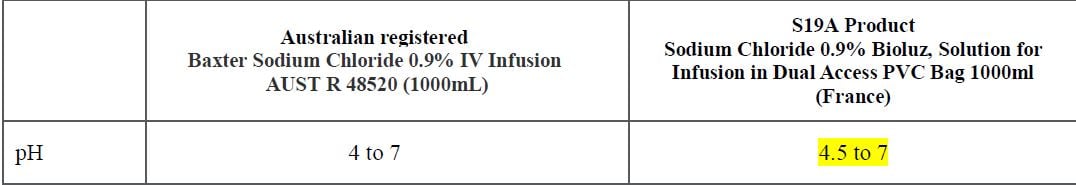
Specifications:
- Product: Sodium Chloride IV 0.9% Dual PVC 1000ml
- Size: 1000ml
- UOM: Box of 50
- Image is a sample of product
Warning: Always read the label and follow directions for use.
Please refer to the attached documents.
Request further information on this product
Newsletter
Please enter your email address to subscribe to our newsletters.